Designed for convenience, speed , and “Green Chemistry” with minimal concession.
These kits are designed to emphasize “Green Chemistry” by minimizing quantities and using recycled materials.
The kits are designed for teachers to purchase one time and reuse the materials every year.
These kits are specifically designed and exclusive to mini-concepts.
All kits are sold exclusively from mini-concepts direct.
Examples of change is not using typical Burets, Pipets, Buret Stands, and Pipet Pumps as tools for measuring volume .Minimize flames with 30 students in a class by using alternative heating sources. Minimize Waste and stick to “Green Chemistry” methodology by reducing quantities to perform and get the same results. By adjusting the methodology more lab time can be used to develop the more time consuming Guided Inquiry Based Labs.
to order by Purchase order
Mail Purchase Order to
Mini Concepts, 67 Fassitt Drive, Mahopac, N.Y. 10541
Mini Concepts is also a NYC vendor with vendor # MAR019000 01.
Subsidiary of Marsilio Langella Inc.
Recommended AP Labs and Associated Topics from College Board
|
Recommended Experiment |
Aligned 2019-2020 Topic |
|
Use the absorption of light to determine the identity and/or concentration of an analyte in solution. Beers Law analysis of cobalt chloride solution Beers law analysis of Blue Dye mixture Spectroscopy determination of the percent of salicylic acid in Aspirin Guided Inquiry |
3.11 |
|
Use differences in intermolecular forces to separate a mixture into its components or to determine the identity of components of a mixture. |
2.1/2.2/3.2/3.7/3.9 |
|
Determine the formula of a compound by measuring the stoichiometry of the reaction of that compound with known reagents. |
4.4/4.5 |
|
Use gravimetric analysis to determine the amount of an analyte in a mixture. |
1.1/1.3 |
|
Use titration to determine the concentration of an analyte in a solution. |
4.2/4.6 |
|
Determine the rate law of a chemical reaction. |
5.3 |
|
Use calorimetry to determine the change in enthalpy of a process. |
6.4 |
|
Build electrochemical cells to determine the |
4.7/9.7 |
|
Explore the impact of disturbances on an equilibrium system. Lechatelier’s Principle using Cobalt complexes and Carbon Dioxide Chemical Equilibrium Lab |
7.9/7.10 |
|
Use titration to characterize an acid or base solution. |
8.5 |
|
Prepare a buffer solution. The determination of the Ionization Constant of an Indicator using Spectroscopy |
8.8/8.9/8.10 |
Our Lab List and Procedures
|
1 |
Unit Conversions Thermocouple Temperature Recording Kit- STEM1 |
40 Min |
|
2 |
Another Column Chromatography Experiment Use of Volumetric Glassware Simple Distillation Kit Column Chromatography Suction Apparatus |
40 Min |
|
3 |
The Scientific Method Guided Inquiry Based Science Practice 6 |
40 Min |
|
4 |
Excitor LED Box -Relationship between Energy and WavelengthLed Plancks Constant Demo Kit-LED Exciter Box – Item # 400 |
40 Min |
|
5 |
Graphical Determination of Planck’s Constant https://mini-concepts.com/product/plancks-constant-probe-kit-for-vernier-lab-quest-item-650/ Planck’s Constant Probe Kit for Vernier Lab Quest Item# 650 |
40 Min |
|
6 |
Spectroscopy of Gases Using Discharge Tube Determine the emission Spectrum of Various known gases from given gas discharge tubes. |
40 Min |
|
7 |
Flame Test of the Elements and Elemental Ions |
40 Min |
|
8 |
Para magnetism using electron configuration and magnetic attractions Paramagnetism Electron Configuration Lab |
40 Min |
|
9 |
Using Microsoft Excel for Analysis of Periodic Tendencies and Meet the Elements Lab Exceptions to Periodicity |
40 Min |
|
10 |
Chemical Reactions using Crystal Growth.( Guided Inquiry) Single replacement Reactions Being Developed |
40 Min |
|
11 |
Molecular Modeling Using modeling foam balls, sticks and MolView Predict the shapes of molecules by building a model of the molecule with a molecular modeling kit and applying the Valence Shell Electron Pair Repulsion Theory Being Developed |
40 Min |
|
12 |
Inter-molecular attractions Lab Determine relationship of capillary action to polarity IMF Kit # 99 |
40 Min |
|
13 |
Molecular Interactions Guided Inquiry Random miscibility determines solubility of given solutes and solvents Being Developed |
40 Min |
|
14 |
Surfactants Tri-esters Being Developed |
40 Min |
|
15 |
Banana Oil and Oil of Wintergreen |
40 Min |
|
16 |
Polymerization, Polymer identification and Elastomers Synthesis of Polyurethane |
40 Min |
|
17 |
Aspirin Synthesis and Analysis Esterification Synthesis Being Developed |
40 Min |
|
18 |
Experimental Determination of Empirical formula of Manganese Chloride |
40 Min |
|
19 |
40 Min |
|
|
20 |
40 Min |
|
|
21 |
Experimental Determination of the Molar Mass of Aluminum Determination of Molar Mass of Aluminum Kit # 800 |
40 Min |
|
22 |
Heat (Enthalpy) of a Reaction: Mg-HCl-Water System https://drive.google.com/file/d/1-KQBokzxmaAkbHYZWmp6E0VYJ1jb48s5/view?usp=sharing |
40 Min |
|
23 |
40 Min |
|
|
24 |
40 Min |
|
|
25 |
Calculate the needed volume of oxygen to react with given volume of gases, determine the heat of the reaction, to determine the amount of work and heat produced from the reaction. Relate energy changes associated with a chemical reaction to the enthalpy of the reaction, and relate energy changes to PΔV work. |
40 Min |
|
26 |
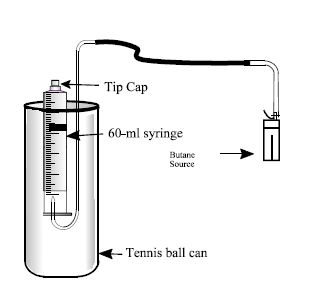
Determining the Molecular Mass of Butane Determine Molar Mass Of Butane Kit item # 700 |
40 Min |
|
27 |
Avogadro’s Law |
40 Min |
|
28 |
Determination of identity of unlabeled bottles from stock room Guided Inquiry Covalent Bonding |
40 Min |
|
29 |
Preparation of Standardized Salicylic Acid Solution |
40 Min |
|
30 |
Predicting Double Replacement Reactions in Solutions based on Solubility Rules |
40 Min |
|
31 |
Beers Law analysis of cobalt chloride solution or Use the absorption of light to determine the identity and/or concentration of an analyte in solution. Beers Law analysis of cobalt chloride solution Beers law analysis of Blue Dye mixture Beer’s Law Kit |
40 Min |
|
32 |
Spectroscopy determination of the percent of salicylic acid in Aspirin Guided Inquiry Students determine method of determine Salicylate Method of preparation of stock solutions Determine percent salicylic acid in expired Aspirin tablet. Being Developed |
40 Min |
|
33 |
Using Freezing-Point Depression to Find Molecular Weight-SIM |
40 Min |
|
34 |
The Kinetics of a Bleach Reaction Graphical Kinetics Analysis Students develop procedure to determine graphical analysis of food coloring decomposition using Bleach Pseudo Rate Food Coloring Rate Law using Graphical Analysis |
40 Min |
|
35 |
Guided Inquiry Kinetics of decomposition of Hydrogen Carbonate Nature of reactants Kinetics Guided Inquiry |
40 Min |
|
36 |
Kinetics of the Acid Decomposition of Thiosulfate Graphical determination of order Initial Rate Method Lab- Kinetics of the Acid Decomposition of Thiosulfate |
40 Min |
|
37 |
Determination of Solubility Constant of Calcium Hydroxide using Micro-titration techniques– Determination of Equilibrium Constant of Saturated Calcium Hydroxide Solution |
40 Min |
|
38 |
Lechatelier’s Principle using Cobalt complexes and Carbon Dioxide Chemical Equilibrium Lab Common Ion Effect Aqueous Carbon Dioxide Equilibrium Kit # 606 |
40 Min |
|
39 |
Determination Of Equilibrium Constant for a system at Equilibrium Column Ion Exchange Determination of Equilibrium Constant Lab using Column Exchange |
40 Min |
|
40 |
Preparation of Sodium Hydroxide Solution and Standardization using a primary standard Endpoint Demo Video |
40 Min |
|
41 |
Guided Inquiry Determination of Molecular Weight and Ka of an Unknown Organic Acid pH Probes |
40 Min |
|
42 |
Types of Salts |
40 Min |
|
43 |
The determination of the Ionization Constant of an Indicator using Spectroscopy Prepare Buffer Solutions Being Developed |
40 Min |
|
44 |
Redox Standardization of HCl using Potassium Iodate – https://mini-concepts.com/product/reduction-oxidation-titration-kit-55/ |
40 Min |
|
45 |
Guided Inquiry Analysis of Household Peroxide https://mini-concepts.com/product/miniscale-hydrogen-peroxide-analysis-kit/ |
40 Min |
|
46 |
Voltaic cell and Nerst Equation Lab. Variations of concentration effects Sketch of an electrochemical cell for all the cells created Include each half-cell, the salt bridge, the electrodes and solutions, the voltmeter leads, the voltmeter and a switch in your drawing |
40 Min |
|
47 |
Electrolysis of Aqueous Solutions Lab A list of all the particles (ions and molecules) present in the U-tube before electrolysis https://mini-concepts.com/product/electolysis-of-aqueous-solutions-lab/ |
40 Min |
|
48 |
Determine the number of faradays, coulombs, and current used to coat a leaf with copper |
40 Min |
|
49 |
Electrolysis of Sodium Sulfate using Micro Hoffman apparatus Given experimental research using syringes to collect gases over water, Atmospheric pressure and room temperature,collect an unknown volume of gas generated using an electrolytic cell,determine the systems net ionic equation, moles of gas , moles of electrons used to generate gas , Determine amperes used in the experiment |